Silicon’s atomic structure makes it an extremely important semiconductor...
【The relationship between silicon and semiconductors】
Silicon’s atomic structure makes it an extremely important semiconductor, and silicon is the most important semiconductor in the electronics and technology sector. Addition of an element such as boron, an atom of which can be substituted for a silicon atom in the crystal structure but which provides one less valence electron than silicon, allows silicon atoms to lose electrons to it. The positive holes created by the shift in electrons allow extrinsic semiconduction of a type referred to as positive. Addition of an element such as arsenic, an atom of which can also be substituted for a silicon atom in the crystal but which provides an extra valence electron, releases its electron within the lattice. These electrons allow semiconduction of the negative type. Highly purified silicon, doped with such elements as boron, phosphorus, and arsenic, is commonly known as a silicon wafer and is the basic material used in computer chips, integrated circuits, transistors, silicon diodes, liquid crystal displays, and various other electronic and switching devices.
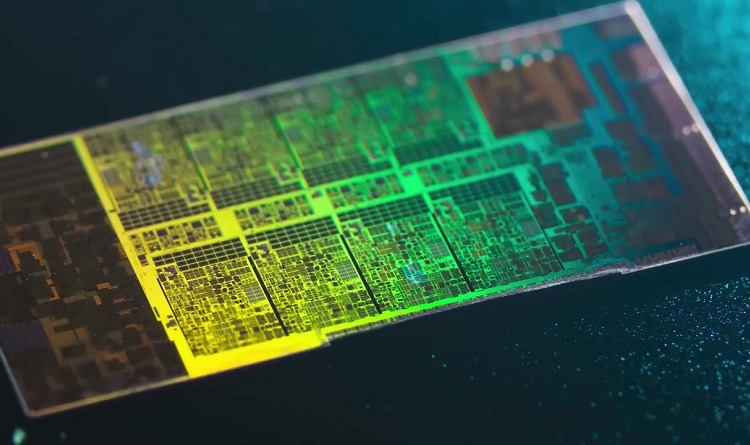
#ic #icchip #semiconductor #technology #electronic #integratedcircuit #manufacturing #electronicchip #electroniccomponents